The fight against cancer takes many shapes and forms. Dave never misses a marathon that supports cancer efforts. Marisol grew out her hair, so she can donate it to children undergoing chemotherapy. When Lisa’s late husband passed, her family started a campaign in his name to raise funds for cancer research. What can you do to support the fight against cancer?
The pivot in a cancer patient’s path toward survival is often clinical trials. Without clinical trials, countless patients would be without effective treatment.
Do you have to have cancer to participate in a trial? Usually yes, but not always. Like any clinical trial for any condition, healthy patients are required for preventative and comparative studies.
We hope that more healthy people and cancer patients alike will participate in clinical trials and contribute to the search for more effective detection, diagnostics, treatments, and therapy.
What are clinical trials?
IN THIS ARTICLE
Clinical trials are studies on people of new treatments, medications, or devices that have proven in laboratory research to be more effective than currently available treatments.
Why participate in a clinical trial?
Every year millions of patients seek and participate in clinical trials in the United States alone. Every patient has their own personal or medical reasons to join a study. Here are some of the most common ones.
Latest Treatment
As doctors and researchers are working together around the world to discover new and better ways to help people lead healthier lives, the current widely used treatments are left in the shadows as comparably outdated or less effective.
In the United States, in particular, the Federal Drug Administration (FDA) is known for its stringent adherence to protocols that render the application process longer than some cancer patients might hope. Clinical trials are a way for these patients to get the treatment they want now.
More Options
While many patients are driven to clinical trials to get the most up-to-date options, still others have limited options, if any. Countless conditions have no treatments until now. In some cases, available treatments may not be effective for some patients. With the many types of cancer, there simply aren’t treatments for everyone. For many cancer patients, clinical trials represent their only hope for recovery or a pain-free life.
Helping Others
Perhaps the most common reason that unites all clinical trial participants is the desire to help others. Every person who participates in a clinical trial is contributing to getting better medications and devices out to the public faster.
Healthy individuals with a known medical condition in the family, no medical history, and current patients alike feel the personal pleasure of knowing that their time and efforts are potentially helping thousands of people in the future.
Cost
Research sponsors generally cover much of the cost of the research. Expensive medications—which may not be covered fully by a patient’s insurance—may be covered entirely as part of the research study. By federal law, insurance companies are required to cover FDA approved clinical trials, with some conditions. The National Cancer Institute covers the topic of patient care costs and clinical trials.
Quality of Care
As per the research objectives, these studies aim to determine not just the efficacy of the new drugs, but also their side effects. Patients may find that their health is monitored more carefully for effective change in the condition or side effects, than with normal medications that have been on the market for tens of years.
Are Clinical Trials Safe?
The question that everyone new to trials asks—and rightly so—is “Is it safe?” The simple answer is “Generally, yes.”
Before a study can start recruiting people, they must first earn approval from an Institutional Review Board (IRB). The IRB committee is comprised of a diverse team of scientists, doctors, activists, etc. Their purpose is to review and monitor any biomedical research that involves testing on people. The IRB is authorized to approve/disapprove, request modifications, or terminate a study. They also review the informed consent documents and brochures to protect the rights of all volunteers.
As you will see in a moment, treatments in Phase IV have already gone through a battery of tests—from the test tube, to animals, to people—and have been approved by the FDA for testing in large scale public clinical trials.
However, it is important to keep in mind that the purpose of the clinical trial is to study the new treatment on a larger sample population to determine if there is a segment for whom the medication is not effective or presents unexpected side effects. As such, no pharmacist or doctor will unequivocally declare any medication or treatment option is safe for everyone.
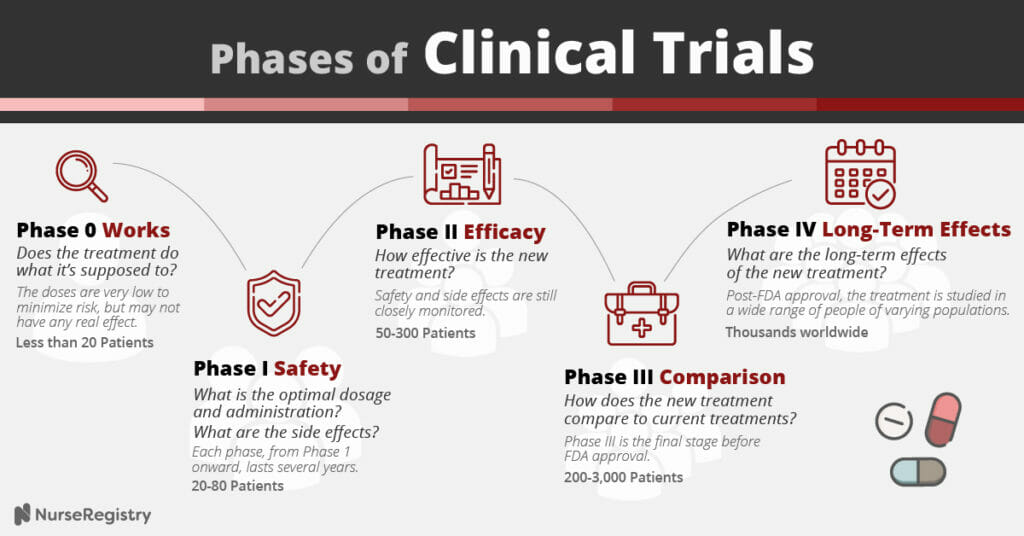
What are the Phases of Clinical Trials?
Do you need to know the various phases of clinical trials? Yes. When you start searching for clinical trials, you’ll be asked—whether in an online search tool or in person—what stage you’re interested in. You’ll want to research the options and discuss them with your doctor.
Phase 0: Works
Phase 0 is the least common because it involves so few patients. These trials set out to determine if the medication does what it’s supposed to at the most basic level.
Researchers hope to answer questions like “Does the medication reach the tumor?” The medications are used in extremely small dosages, so as to be safe. As a result, oftentimes the doses are not high enough to have any real effect. (Study size: less than 20 patients)
Phase I: Safety
In Phase I, researchers aim to determine optimal dosage, administration, and side effects in a small group of patients. (Study size: 20-80 patients)
Phase II: Efficacy
In Phase II trials, the focus is on how effective the new medication is, but the safety and side effects are still closely monitored. (Study size: 50-300 patients)
Phase III: Comparison
Phase III is the final phase before FDA approval. The new treatments are compared to currently available treatments and sometimes placebos. (Study size: 200-3,000 patients)
Phase IV: Long-term Effects
Post-FDA approval, these studies aim to study the long-term effects of medications that are in use and deemed safe by the FDA. (Study size: thousands worldwide)

Stanford Cancer Institute Clinical Trials
Every day, Stanford researchers are in the news for new discoveries. Behind the scenes, there are thousands of volunteers who work to make these dreams come true. But within Stanford, the clinicians and staff all know who the true unsung heroes are.
Some of these heroes are celebrated on a dedicated page on the Stanford Cancer Institute website. Let’s introduce you to a few….(taken from the Stanford Cancer Clinical Trial Stories)
Noah | Neuroblastoma
Cathy shares her heart-wrenching turned heart-warming son Noah’s journey for neuroblastoma treatment. Watch this video for an inspirational story and to learn more about participating in a cancer clinical trial for neuroblastoma at the Stanford Cancer Institute.
Dan | Leukemia
Dan Rosenbaum, a Stanford cancer clinical trial participant, relaxes with his furry pal. After receiving new targeted treatments for his chronic lymphocytic leukemia (CLL), Dan noticed a difference almost immediately. “I saw a marked improvement in my symptoms within two weeks of starting treatment, with little or no side effects,” Dan said. “It’s so unbelievable it is almost hard to talk about.” Read more…

Neil | Lung Cancer
Neil shares his good story about lung cancer. And, Neil delivers. This truly is a good story worth listening to whether or not you’re considering participating in a cancer clinical trial for lung cancer at the Stanford Cancer Institute, as Neil had.
Clinical Trials During the COVID-19 Pandemic
Patients have always found comfort in Stanford’s premier innovations and compassionate care. Now, Stanford takes extra measures to ensure the safety and well-being of all participants in their clinical trials. These are some of those measures, as listed on their website.
- Remote consenting of new and ongoing participants
- Offering virtual office visits, whenever possible
- Directly shipping orally administered study drugs to patients, whenever possible
How to Find a Cancer Clinical Trial at Stanford
With over 250 active clinical trials at any given time, the Stanford Cancer Institute is always recruiting. These trials cover a range of services and purposes including diagnostic, preventative, and therapeutic studies.
To find a trial, you can search their website—which we will visit in a moment— sign up for email alerts, download their app, or simply email or call them to talk to someone live.
Search the Website
Stanford provides two ways to search for a clinical trial on their website: by condition, and by eligibility requirements.
It’s worth noting here that not everyone can participate in any trial. There will be a screening process to ensure the trial is a good match for you and for the research objectives of the study.
Search by Condition
From this page, you can select a condition and/or enter keywords for your search.
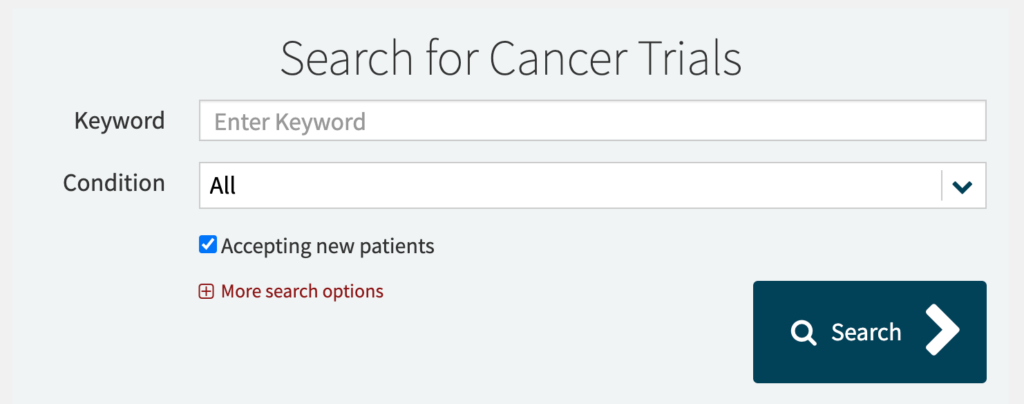 For rare forms of cancer, like eye cancer, for example, you may find just a couple of studies. For more prevalent cancers like breast cancer, you’ll find well over twenty studies and may want to narrow down your search.
For rare forms of cancer, like eye cancer, for example, you may find just a couple of studies. For more prevalent cancers like breast cancer, you’ll find well over twenty studies and may want to narrow down your search.
By clicking on “More search options,” you can narrow down your search by some simple eligibility criteria like age, gender, or phase of the study.
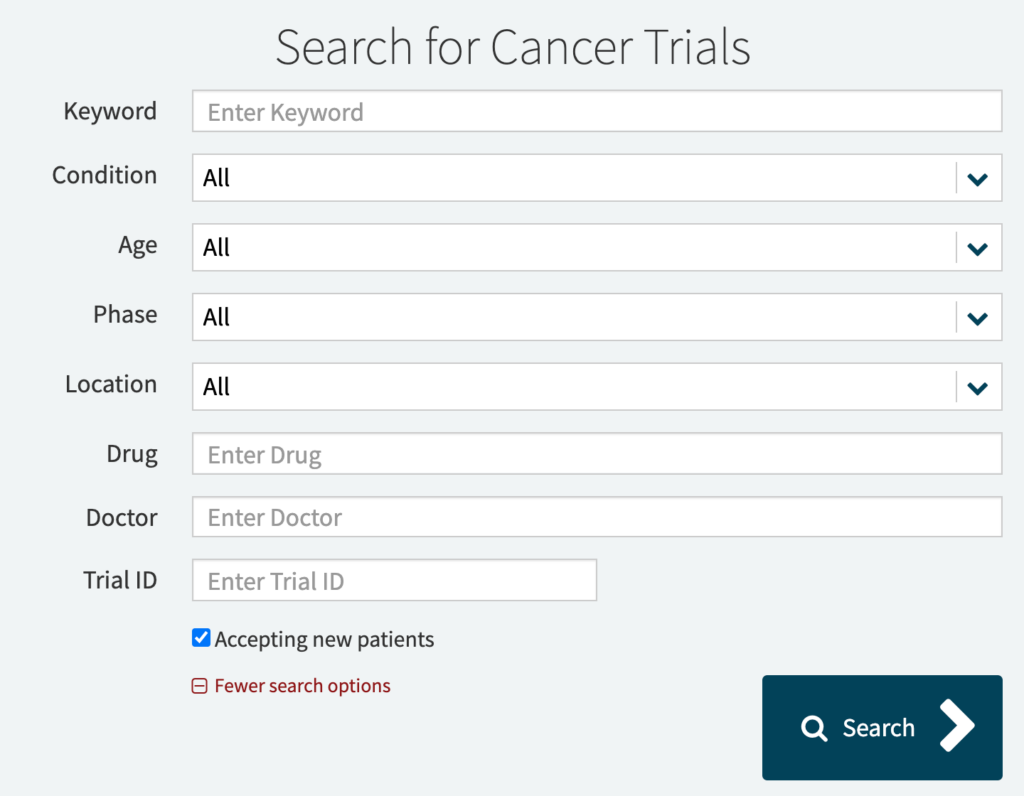 If you’d like to browse through the list of actively recruiting studies instead, you can do so here.
If you’d like to browse through the list of actively recruiting studies instead, you can do so here.
Search by Eligibility
The search by eligibility requirements can be tedious. Here are the steps simplified.
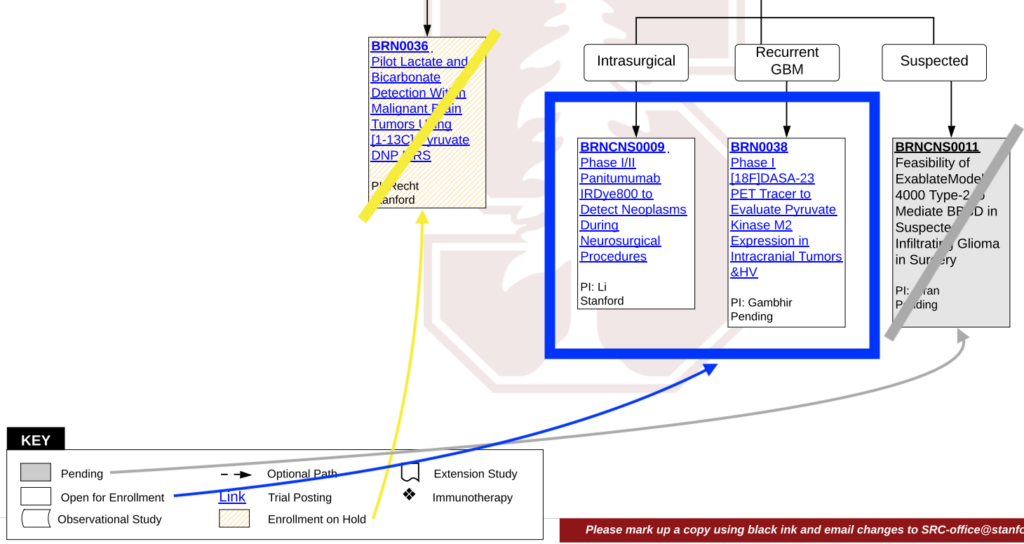
- First, select a condition or therapy from this list of conditions.
- Several categories will appear, like this one.

- From there, you can download the flowchart as a PDF.
Reading the flowchart, you will want to look into the clinical trials that are featured in a white rectangle. (Gray rectangles are pending, and yellow rectangles are on hold, so you might want to come back later to check on their status).
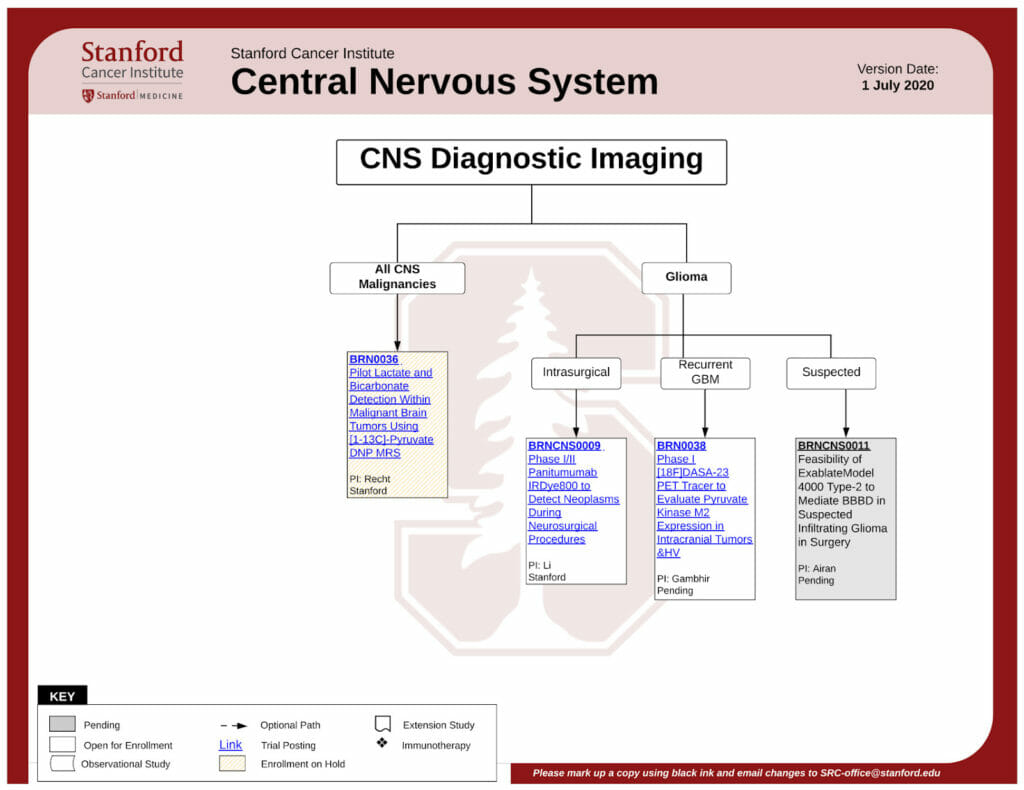
- To learn more about a particular study, click on the blue link, and you will be taken to the trial’s dedicated page. There, you’ll find detailed information about the study’s purpose, investigators, phase, and, of course, the eligibility criteria (both inclusion and exclusion criteria).
Sign up for Email Alerts
You can sign up for email alerts to hear about trials as soon as they are open to patients. Simply indicate the condition and provide your email address here.
 Download the App
Download the App
You can search clinical trials on the SCI Cancer Clinical Trials App, available on the App Store for iPhone and iPad.

Contact Them
You can call or email the Stanford Clinical Trials office to learn more about the trials. Please note that while the office may help you find a trial, they do not provide doctor’s advice or a second opinion.
Phone: 650-498-7061
Email: ccto-office@stanford.edu
Note: This service only provides general information about how to find our trials. It does not provide a doctor’s advice or a second opinion.
What next?
With the healthcare professionals at the Stanford Cancer Institute, you are in good hands. Talk with your oncologist about opportunities to join a clinical trial.





